The Valley Hospital Among First to Participate in Impella ECP Pivotal Trial
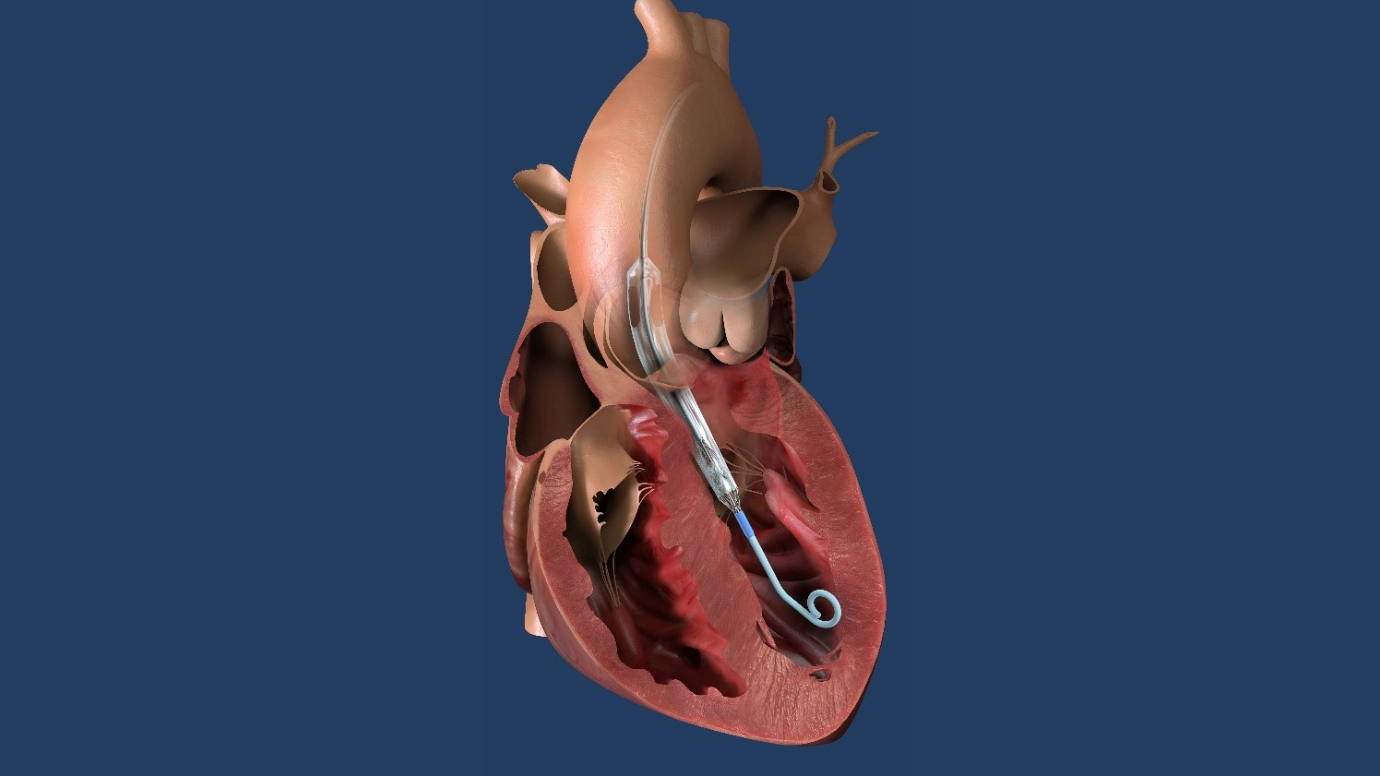
RIDGEWOOD, NJ, May 1, 2023 – The Valley Hospital is one of the first sites in the Impella ECP Pivotal Trial sponsored by Abiomed, part of Johnson & Johnson MedTech.
The Valley Hospital, Hackensack University Medical Center, and Morristown Medical Center enrolled the first New Jersey patients into the trial expanding enrollment to the second state in the United States. Up to 256 patients will be enrolled in the pivotal study and receive Impella ECP support during high-risk percutaneous coronary intervention (PCI) procedures.
The Impella ECP was designed to improve procedural safety and efficacy for patients undergoing coronary revascularization, procedures to restore blood flow to areas of the heart that are not receiving adequate blood supply, and need require additional blood flow support during their procedure.
Rajiv Tayal, MD, MPH, Director of the Cardiac Catheterization Laboratory and Structural Heart Program at The Valley Hospital, and Hussein Rahim, MD, interventional cardiologist, performed the high-risk PCI cases with Impella ECP support at The Valley Hospital.
“At Valley, we regularly perform high-risk percutaneous coronary intervention procedures on patients who meet the definition of ‘high-risk,’ whether that be due to age, previous medical history, or other factors,” said Dr. Tayal. “Our participation in this study puts us among the top innovators in the field who are looking to improve patient outcomes for these procedures.”
“Participating in this study is a great opportunity for Valley. It helps us to further patient care and continue to expand our armamentarium of treatments for PCI cases,” said Francis Kim, MD, Associate Director of the Cardiac Catheterization Laboratory at The Valley Hospital.
At Morristown Medical Center, PCI procedures with Impella ECP support were performed by interventional cardiologists Amir Masoumi, MD, and Dimitrios Karmpaliotis, MD. Interventional cardiologists Ankit Patel, MD, and Haroon Faraz, MD, performed the Impella ECP cases at Hackensack University Medical Center.
The Food and Drug Administration (FDA) approved the Impella ECP early feasibility study (EFS) in June 2020. Impella ECP received an FDA breakthrough device designation in August 2021, demonstrating that Impella ECP meets the FDA’s stringent requirements for a breakthrough device. Impella ECP is the first mechanical circulatory support device to use the EFS pathway as a steppingstone to a US pivotal study. Additional details about the Impella ECP pivotal study are available here.
Impella ECP is an investigational device limited by federal law to investigational use only.
Photo Caption: Impella ECP pump in heart illustration. Photo courtesy of Abiomed, Inc.


What's Related